Crispr Cancer Trial Success Paves The Way For Personalized Treatments
The CRISPRCas9 complex can precisely cut DNA .Credit: Alfred Pasieka/Science Photo Library
A small clinical trial has shown that researchers can use CRISPR gene editing to alter immune cells so that they will recognize mutated proteins specific to a persons tumours. Those cells can then be safely set loose in the body to find and destroy their target.
It is the first attempt to combine two hot areas in cancer research: gene editing to create personalized treatments, and engineering immune cells called T cells so as to better target tumours. The approach was tested in 16 people with solid tumours, including in the breast and colon.
Landmark CRISPR trial shows promise against deadly disease
It is probably the most complicated therapy ever attempted in the clinic, says study co-author Antoni Ribas, a cancer researcher and physician at the University of California, Los Angeles. Were trying to make an army out of a patients own T cells.
The results were published in Nature and presented at the Society for Immunotherapy of Cancer meeting in Boston, Massachusetts on 10 November.
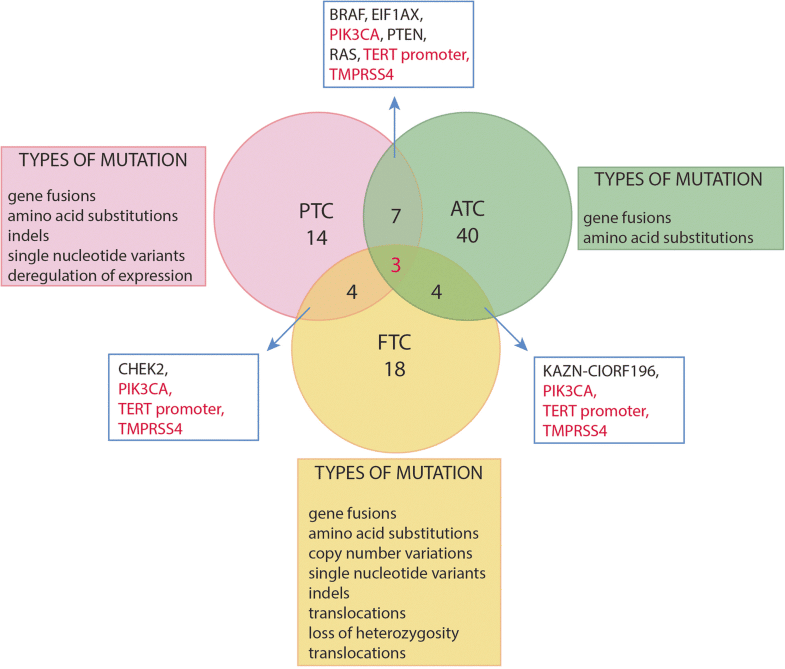
Ras Oncogenes And Ptc
RAS, which is upstream of BRAF, is a family of GTP-binding proteins that regulate cell growth via the MAPK and PI3K-AKT pathways. Almost one-third of human tumours are presented with RAS mutations 48. Mutations of RAS were initially reported in thyroid cancer in 1988 66. They are found in a wide variety of thyroid tumours including follicular adenomas, follicular carcinomas, poorly differentiated carcinomas, undifferentiated carcinomas as well as papillary carcinomas. Three members of the RAS gene family have been shown to be mutated in thyroid cancer. The most common RAS mutations were detected in the NRAS gene, followed by HRAS, and least frequently, KRAS67. However, later it became clear that the RAS mutations are predominantly related to poorly differentiated thyroid carcinomas and anaplastic thyroid cancers than PTC 68, 69, which suggests the role of RAS is more inclined to the progression rather than the initiation of tumours. In a recent report of 199 cases of non-invasive follicular thyroid neoplasm with papillary-like nuclear features, more than half were apparently attributed to RAS mutations 70.
Are Harmful Variants In Brca1 And Brca2 More Common In Certain Racial/ethnic Populations Than Others
Yes. The likelihood of carrying an inherited mutation in BRCA1 or BRCA2 varies across specific population groups. While the prevalence in the general population is about 0.2%0.3% , about 2.0% of people of Ashkenazi Jewish descent carry a harmful variant in one of these two genes and the variants are usually one of three specific variants, called founder mutations. Other populations, such as Norwegian, Dutch, and Icelandic peoples, also have founder mutations .
Different racial/ethnic and geographic populations also tend to carry different variants in these genes. For instance, African Americans have BRCA1 variants that are not seen in other racial/ethnic groups in the United States . Most people of Ashkenazi Jewish descent in the United States who carry a BRCA variant have one of three specific variants . In the Icelandic population, a different variant in BRCA1 is common among those who inherit a mutation in BRCA1.
Don’t Miss: What Can I Take For Thyroid Pain
Rare Germline Mutations In Families With Non
Non-syndromic familial non-medullary thyroid cancer accounts for 95% of FNMTC cases. The genetic risk factors of non-syndromic FNMTC are poorly understood compared to familial NMTC associated with hereditary syndromes . In addition to FOXE1, HABP2, NRG1, SRGAP1, DIRC3, TITF1/ NKX2.1 and PTCSC3, multiple other genes and chromosomal loci have been linked to families affected by non-syndromic FNMTC in linkage studies and/or whole-exome/whole-genome sequencing studies. The identified mutations are present in only a subset of FNMTC kindreds and require further validation studies. Table summarizes multiple studies that investigated the genetic component of FNMTC in families with NSFNMTC.
Table 7 Genes and chromosomal loci linked to non-syndromic familial non-medullary thyroid cancer
Gpx4 Genetic Silencing In Vitro
The lentiviral plasmids, either non-targeted vector or pLKO.1-shGPX4 plasmid DNAs were transfected along with packaging and envelop plasmids psPAX2 and pMD2.G into HEK293T cells. The non-targeted or pLKO.1-shGPX4 lentiviral particles were transiently transduced into K1 cells. After 72 h, cells were harvested, washed, and lysed in lysis buffer and Western blot analysis was performed. GPX4 knockdown levels were confirmed using a GPX4 antibody. All methods involving human material were carried out in accordance with the Declaration of Helsinki guidelines and regulations.
Read Also: Doctors Who Specialize In Thyroid
Retevmo Has Been Shown To Shrink Tumors In The Majority Of People With Other Ret
of the 19 people who had prior cancer treatments§ had an objective response
- Responses lasted a median of 18.4 months
of the 8 people who had not received certain standards of care|| had an objective response
- Median length of response has not yet been reached, as the trial is ongoing
Median is the middle number in a set of numbers.§Radioactive iodine in addition to other systemic therapy, including lenvatinib and/or sorafenib.||Not treated with systemic therapies other than radioactive iodine.
SELECT SAFETY INFORMATION
RETEVMO may cause serious side effects, including:Bleeding problems: RETEVMO can cause bleeding, which can be serious and may lead to death. Tell your doctor if you have any signs of bleeding during treatment, including:
- vomiting blood or if your vomit looks like coffee-grounds
- pink or brown urine
- red or black stools that look like tar
- coughing up blood or blood clots
- unusual bleeding or bruising of your skin
- menstrual bleeding that is heavier than normal
- unusual vaginal bleeding
- nose bleeds that happen often
- drowsiness or difficulty being awakened
The Genetics Of Cancer
Genetic changes that cause cancer can be inherited or arise from certain environmental exposures. Genetic changes can also happen because of errors that occur as cells divide.
Yes, cancer is a genetic disease. It is caused by changes in genes that control the way cells grow and multiply. Cells are the building blocks of your body. Each cell has a copy of your genes, which act like an instruction manual.
Genes are sections of DNA that carry instructions to make a protein or several proteins. Scientists have found hundreds of DNA and genetic changes that help cancer form, grow, and spread.
Cancer-related genetic changes can occur because:
- random mistakes in our DNA happen as our cells multiply
- our DNA is altered by carcinogens in our environment, such as chemicals in tobacco smoke, UV rays from the sun, and the human papillomavirus
- they were inherited from one of our parents
DNA changes, whether caused by a random mistake or by a carcinogen, can happen throughout our lives and even in the womb. While most genetic changes arent harmful on their own, an accumulation of genetic changes over many years can turn healthy cells into cancerous cells. The vast majority of cancers occur by chance as a result of this process over time.
Read Also: Natural Medicine For Low Thyroid
Mutual Exclusivity Between Braf Mutation And Other Common Genetic Alterations In Thyroid Cancer
BRAF mutation and RET/PTC rearrangements may act at steps that are different but close in their shared oncogenic pathway, resulting in conventional PTC, whereas ras mutations and RASSF1A methylation may act at different but related steps along their shared oncogenic pathway resulting in FTC and follicular-variant PTC. Although thyroid tumorigenesis caused by these genetic and epigenetic alterations may all involve the MAP kinase pathway, each of these genetic and epigenetic alterations, particularly those that act in this pathway at a step proximal to Raf kinase, may involve additional signaling pathways. For example, the phosphoinositide 3-kinase/Akt pathway, which is known to also play an important role in thyroid tumorigenesis, can be activated by Ras or RET/PTC . This may partially explain the distinct characteristics of different subtypes of thyroid cancer that harbor different genetic and epigenetic alterations.
How Can I Find Out What Genetic Changes Are In My Cancer
If you have cancer, a different type of genetic test called a biomarker test can identify genetic changes that may be driving the growth of your cancer. This information can help your doctors decide which therapy might work best for you or if you may be able to enroll in a particular clinical trial. For more information, see Biomarker Testing for Cancer Treatment. Biomarker testing may also be called tumor profiling or molecular profiling.
Biomarker testing is different from the genetic testing that is used to find out if you have an inherited genetic change that makes you more likely to get cancer. Biomarker testing is done using a sample of your cancer cellseither a small piece of a tumor or a sample of your blood.
In some cases, the results of a biomarker test might suggest that you have an inherited mutation that increases cancer risk. If that happens, you may need to get another genetic test to confirm whether you truly have an inherited mutation that increases cancer risk.
Recommended Reading: Diabetes And Thyroid Center Of Ft Worth
High Prevalence Specificity And Oncogenic Role Of The T1799a Braf Mutation In Ptc
Numerous studies have consistently shown a high prevalence of BRAF mutation in thyroid cancer, ranging from 29 to 83% . The BRAF mutation found in thyroid cancer is almost exclusively the T1799A transversion mutation in exon 15. This mutation is a somatic mutation in sporadic thyroid cancers and was found not to be a germ-line mutation in a large series of familial PTCs . The only other BRAF mutation reported in thyroid tumors was the K601E mutation found in two benign thyroid adenomas and three follicular-variant PTCs . The mutations in exon 11 of the BRAF gene found in other human cancers were not found in thyroid cancer . A rare but interesting genetic alteration that can also cause constitutive activation of BRAF is the recently reported in vivo fusion of the BRAF gene with AKAP9 gene through a paracentric inversion of the long arm of chromosome 7. This results in a recombinant AKAP9–BRAF oncogene, which appears to occur in PTCs induced by radiation exposure and results in the loss of the autoinhibitory regulatory domains of BRAF and hence constitutive activation of the kinase .
Detection Of Braf Mutations
Mutations of BRAF reported recently in melanomas and colorectal cancers are confined to exons 11 and 15 . DNA samples were screened by SSCP for mutations within these regions, as well as sequencing of gel-extracted and/or whole-sample PCR products. Primer pairs were designed flanking BRAF exons 11 and 15, respectively. PCR primer sequences were as follows: exon 11: 5â²tctgtttggcttgacttgacttt 3â² and 5â²catgccactttcccttgtagac 3â² and exon 15: 5â²aaactcttcataatgcttgctctg 3â² and 5â²ggccaaaaatttaatcagtgga 3â². Amplifications were carried out for 35 cycles with annealing temperatures optimized for each primer pair. Twenty five-μl PCR reactions were performed on 100 ng genomic DNA, 7.5 pmol of each primer, 100 μm deoxynucleoside triphosphates, 5 μCi dCTP, 1.5 mm MgCl2, Platinum TaqDNA polymerase high fidelity , and buffer. SSCP analysis was performed using a method reported previously . The PCR reaction mixture was diluted in DNA gel-loading buffer , denatured by incubating at 94 C for 5 min, placed on ice, and loaded onto a 0.6% mutation detection enhancement gel solution with 10% glycerol. Gels were run with 0.6à Tris-borate EDTA buffer at 8W for 7â10 h at room temperature. Autoradiography was performed with an intensifying screen at â70°C for 12â24 h. All of the PCR reactions from PTC samples were repeated at least twice.
Read Also: Who Is The Specialist For Thyroid
Brca1 And Brca2 Genes
Everyone has BRCA1 and BRCA2 genes. BRCA stands for BReast CAncer gene. They are important genes that stop the cells in our body from growing and dividing out of control. Doctors call these tumour suppressor genes.
A fault in the BRCA1 or BRCA2 gene means that the cells can grow out of control. This can lead to cancer developing.
Faulty BRCA1 and BRCA2 genes are rare. Only around 1 in every 400 people have a faulty BRCA1 or BRCA2 gene.
Both men and women can have a faulty BRCA1 or BRCA2 genes. People who inherit faulty versions of these genes have an increased risk of developing different types of cancers. This includes:
-
pancreatic cancer
Gpx4 Overexpression Negatively Impacts Overall Survival In Patients With Papillary Thyroid Carcinoma

We assessed the GPX4 enzyme expression levels in human PTC. We queried the TCGA database of human PTC tumor specimens using TIMER 2.0 computational platform. GPX4 was overexpressed in PTC tissue samples relative to 59 benign thyroid control tissues . Patients with normalized GPX4 expression> 0.0 were considered to have GPX4 overexpression in 226 patients. mRNA expression data and survival data were available for 96% of patients in the TCGA database with PTC. Patients with GPX4 overexpression had a reduced 5-year OS of 73% compared to 97% in patients with normal GPX4 expression . These findings indicate that enhanced GPX4 expression is prevalent in thyroid cancer and is associated with reduced 5-yr overall survival.
Figure 1
GPX4 and TfR1 expression levels in papillary thyroid carcinoma. TIMER2.0 data analysis from TCGA database of Papillary Thyroid Carcinomas showing estimated GPX4 mRNA expression levels in thyroid cancer compared to the normal thyroid tissue . . GPX4 overexpression is associated with worse 5-year OS . Viability assay of thyroid cancer cell lines K1, MDA-T32, MDA-T68, TPC-1 and HThF control cells after treatment with RSL3 for 48 h. RT-qPCR for GPX4 and transferrin receptor 1 mRNA levels, normalized to -actin, in K1, MDA-T32, MDA-T68, and TPC-1 cancer cell lines, as well as human thyroid fibroblasts . E Protein levels for GPX4 and TfR1 measured by Western blot analysis.
Read Also: Are Home Thyroid Tests Accurate
Detection Of Ras Mutations
Sixty-seven human tumor samples were analyzed for point mutations in codons 12/13, and 61 of the N-RAS, H-RAS, and K-RAS genes using LightCycler fluorescence melting curve analysis. Briefly, 100 ng of DNA from each tumor was amplified with primers flanking codons 12/13 or 61 of each RAS gene using a hybridization probe format followed by fluorescence melting curve analysis . All of the PCR products that displayed a deviation from normal melting pick were directly sequenced to verify the presence of RAS mutation and detect the exact nucleotide change.
Summary And Future Directions
Further work is needed in the following several areas: the elucidation of the specific molecular and cellular alterations and events that are caused by BRAF mutation and MAP kinase pathway activation in thyroid cancer the possible restoration of the ability of thyroid cancer cells to metabolize iodide by interfering with BRAF mutation-initiated aberrant signaling the improvement of the diagnostic utility of BRAF mutation, possibly through combination with other specific molecular markers for thyroid cancer in conjunction with FNAB, and through the establishment of a BRAF mutation-based blood test the clinical application of the prognostic value of BRAF mutation in guiding the optimal short- and long-term managements of thyroid cancer patients and further preclinical and clinical studies on the therapeutic potential of novel inhibitors of MAP kinase pathway. It is anticipated that rapid advancements in these areas will occur in the next few years.
Table 1
Don’t Miss: Iodine Treatment For Thyroid Cancer
Risk Of Ptc Among Patients With Nodular Goitre
The reported risk of PTC malignancy among patients with benign nodular goitre is not consistent. Earlier studies comparing patients with multiple nodular goitre with those having a single thyroid nodule showed no difference in cancer prevalence 15. Later studies suggested patients with a solitary thyroid nodule carry a higher risk of thyroid cancer than patients with multiple thyroid nodules 16. However, these views are no longer tenable as numerous subsequent studies have reported a significant risk of PTC in the patients with multiple thyroid nodules 17, 18.
PTC is apparently the most common variety of thyroid malignancy that was incidentally detected in patients with benign thyroid goitre 19, 20. The risk of PTC in multinodular goitre is reported to vary from as low as 6% to as high as 21.2% 21, 22. An earlier study by Alevizaki et al. 23 has reported that more than 50% of PTC cases were incidentally detected in elderly patients operated for pre-existing multinodular goitre. Variation in the frequencies of thyroid cancer in Graves’ disease has also been observed, with 0.5% to 18.7% of the patients having PTC 24.
Iodine Intake And Eating Habits
Iodine intake may influence the incidence and prevalence of thyroid disease in general and of thyroid cancer in particular . In fact, iodine deficiency is associated with an increased risk of FTC, whereas chronically high iodine intake may increase the risk of PTC . The prevalence of BRAF mutations was significantly higher in high-iodine content regions when compared with normal-iodine-content regions. In some studies iodine exerts protective effects on thyroid cancer cell lines by attenuating acute BRAF oncogene-mediated microRNA deregulation .
Excess carbohydrate and protein diet and high body weight increase the risk of thyroid cancer . The accumulation of carbohydrate and the impairment of insulin regulation might lead to a deregulation of the PI3K/AKT pathway, disorganization of cell growth and proliferation which has been strongly related to DTC development and progression .
You May Like: Treatment For Thyroid Problems In Cats
What Are Brca1 And Brca2
BRCA1 and BRCA2 are genes that produce proteins that help repair damaged DNA. Everyone has two copies of each of these genesone copy inherited from each parent. BRCA1 and BRCA2 are sometimes called tumor suppressor genes because when they have certain changes, called harmful variants , cancer can develop.
People who inherit harmful variants in one of these genes have increased risks of several cancersmost notably breast and ovarian cancer, but also several additional types of cancer. People who have inherited a harmful variant in BRCA1 and BRCA2 also tend to develop cancer at younger ages than people who do not have such a variant.
A harmful variant in BRCA1 or BRCA2 can be inherited from either parent. Each child of a parent who carries any mutation in one of these genes has a 50% chance of inheriting the mutation. Inherited mutationsalso called germline mutations or variantsare present from birth in all cells in the body.
Even if someone has inherited a harmful variant in BRCA1 or BRCA2 from one parent, they would have inherited a normal copy of that gene from the other parent . But the normal copy can be lost or change in some cells in the body during that persons lifetime. Such a change is called a somatic alteration. Cells that dont have any functioning BRCA1 or BRCA2 proteins can grow out of control and become cancer.